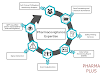
The development of a new drug involves a complex and meticulous process that requires extensive research, rigorous testing, and regulatory compliance. In this article, we will walk you through the step-by-step process that a pharmaceutical company follows to bring a new drug to the market.
Step 1: Discovery and Target Identification
The first step in drug development is identifying a target, such as a specific disease or condition, that the drug aims to address. Scientists conduct extensive research to understand the underlying causes of the disease and identify potential drug targets, such as specific proteins or enzymes.
Step 2: Drug Design and Synthesis
Once the drug target is identified, scientists design and synthesize molecules that can interact with the target and produce the desired therapeutic effect. This process involves creating numerous variations of the molecule and testing their efficacy and safety through computer modeling and laboratory experiments.
Step 3: Preclinical Testing
Before testing the drug on humans, preclinical studies are conducted using laboratory animals to evaluate its safety, efficacy, and potential side effects. These studies help researchers gather important data on the drug's pharmacokinetics, toxicology, and dosage levels, which are essential for determining the appropriate starting dose for human trials.
Step 4: Investigational New Drug (IND) Application
If the preclinical studies yield promising results, the pharmaceutical company submits an Investigational New Drug (IND) application to the regulatory authorities, such as the Food and Drug Administration (FDA) in the United States. The application includes comprehensive data from the preclinical studies, as well as proposed plans for human clinical trials.
Step 5: Clinical Trials (Phases I, II, and III)
Clinical trials are conducted in three phases to evaluate the drug's safety, efficacy, dosage, and potential side effects in human subjects.
- Phase I: A small group of healthy volunteers is given the drug to determine its safety, dosage, and how the body metabolizes it.
- Phase II: The drug is administered to a larger group of patients with the target disease to assess its effectiveness, optimal dosage, and potential side effects.
- Phase III: The drug is tested on an even larger population, typically involving thousands of patients, to further evaluate its effectiveness, monitor side effects, and compare it to existing treatment options.
Step 6: New Drug Application (NDA) Submission
If the results from the clinical trials are favorable, the pharmaceutical company prepares a New Drug Application (NDA) for submission to the regulatory authorities. The NDA includes detailed information on the drug's efficacy, safety profile, manufacturing processes, and proposed labeling information.
Step 7: Regulatory Review
Upon receiving the NDA, regulatory authorities thoroughly review the data and information provided. This review process aims to ensure that the drug's benefits outweigh its risks and that it meets all safety and efficacy standards. The regulatory review process can be lengthy and involves a comprehensive evaluation of the drug's quality, safety, and effectiveness.
Step 8: Approval and Post-Marketing Surveillance
If the regulatory authorities approve the NDA, the drug is granted market authorization, and it can be made available to patients. However, the process does not end there. Pharmaceutical companies are required to conduct post-marketing surveillance to monitor the drug's safety and effectiveness in real-world settings. Any reported adverse effects or unexpected outcomes are closely monitored, and appropriate actions are taken to ensure patient safety.




0 Comments